
Chemistry, 09.07.2019 17:30, megansanders215
Consider the reaction below: a (aq) ↔ b (aq) kc = 2.36 if the reaction is started by placing 0.134 mol of a into 250.0 ml of solution, what will the concentration of a be at equilibrium?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:40, arlabbe0606
Effect of rotenone and antimycin a on electron transfer rotenone, a toxic natural product from plants, strongly inhibits nadh dehydrogenase of insect and fish mitochondria. antimycin a, a toxic antibiotic, strongly inhibits the oxidation of ubiquinol. (a) explain why rotenone ingestion is lethal to some insect and fish species. (b) explain why antimycin a is a poison. (c) given that rotenone and antimycin a are equally effective in blocking their respective sites in the electron-transfer chain, which would be a more potent poison? explain.
Answers: 3

Chemistry, 23.06.2019 00:00, queenpaige2015
Which samples do the atoms have the least kinetic energy
Answers: 2
Do you know the correct answer?
Consider the reaction below: a (aq) ↔ b (aq) kc = 2.36 if the reaction is started by placing 0.134...
Questions in other subjects:



Biology, 05.11.2020 23:40

Biology, 05.11.2020 23:40




Mathematics, 05.11.2020 23:40




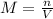
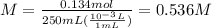
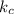 for the equilibrium reaction is:
for the equilibrium reaction is:![k_{c}=\frac{[b]}{[a]}](/tpl/images/0070/2226/ea1a2.png)
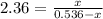

![[a]=0.536-x=0.536-0.376=0.16 M](/tpl/images/0070/2226/22b1e.png)






