
Amixture of babr2 and inert material is analyzed to determine the ba content. first the mixture is dissolved in water. then all of the bromide ion is precipitated as agbr by the addition of an excess of silver nitrate. babr2(> 2agbr(s)+ba(no3)2(aq). in one experiment a 0.7207 g sample of the mixture resulted in 0.6226 g of agbr. determine the percent (by mass) of ba in the mixture

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, junkmailemail42
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 13:00, aleilyg2005
If two objects at different te, peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 22.06.2019 18:30, kate3887
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1
Do you know the correct answer?
Amixture of babr2 and inert material is analyzed to determine the ba content. first the mixture is d...
Questions in other subjects:

Mathematics, 12.04.2021 23:20

Mathematics, 12.04.2021 23:20


Mathematics, 12.04.2021 23:20


Mathematics, 12.04.2021 23:20

Mathematics, 12.04.2021 23:20



English, 12.04.2021 23:20


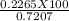 =31.42 %
.
=31.42 %
.



