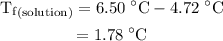Chemistry, 09.07.2019 21:00, genyjoannerubiera
Part a cyclohexane has a freezing point of 6.50 ∘c and a kf of 20.0 ∘c/m. what is the freezing point of a solution made by dissolving 0.925 g of biphenyl (c12h10) in 25.0 g of cyclohexane?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, leslyrivera11
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 01:30, MickeyxX7096
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 06:20, Naysa150724
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 08:30, ebigham5117
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2
Do you know the correct answer?
Part a cyclohexane has a freezing point of 6.50 ∘c and a kf of 20.0 ∘c/m. what is the freezing point...
Questions in other subjects:



Mathematics, 22.01.2022 14:00

History, 22.01.2022 14:00



Mathematics, 22.01.2022 14:00

Mathematics, 22.01.2022 14:00


Mathematics, 22.01.2022 14:00



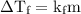 ...... (1)
...... (1)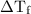 is the change in freezing point.
is the change in freezing point.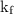 is the freezing point depression constant.
is the freezing point depression constant.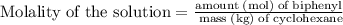 ……. (2)
……. (2)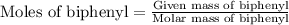 …… (3)
…… (3)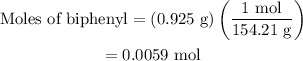
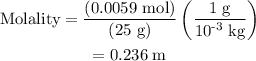
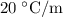 .
.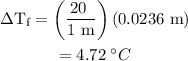
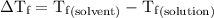 ...... (4)
...... (4)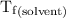 is the temperature of the solvent.
is the temperature of the solvent.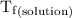 is the temperature of the solution.
is the temperature of the solution.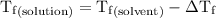 ……. (5)
……. (5)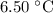 for
for 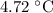 for
for