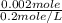Chemistry, 09.07.2019 21:30, brianna4455
Suppose you believe your unknown compound is naoh, and you prepare 20 ml of a 0.1 mol/l solution of it. the stockroom provides a 0.2 mol/l hcl solution as the titrant. what volume of hcl (in ml) is needed to reach the equivalence (stoichiometric) point if your unknown compound is naoh? naoh(aq) + hcl(aq) --> nacl(aq) + h2o(l)

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 05:40, shelbylynn1093
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2

Do you know the correct answer?
Suppose you believe your unknown compound is naoh, and you prepare 20 ml of a 0.1 mol/l solution of...
Questions in other subjects:











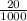 × 0.1
× 0.1