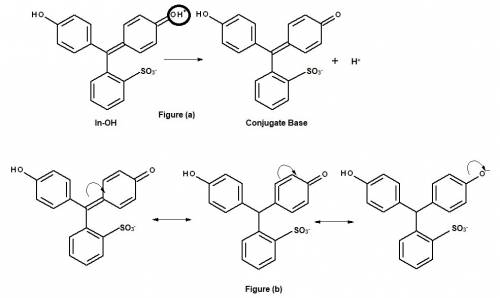
The phenolic indicator (in-oh)has approximately the same pka as a carboxylic acid. which h is the most acidic proton in in-oh? circle or otherwise indicate the most acidic proton. explain why that h is the most acidic proton in in-oh(i. e. what makes its conjugate base well-stabilized)?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, cami30031cami3003
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 22.06.2019 05:00, mahhvelousg97
You mix the pks of succinic acid are 4.21 and 5.64. how many gramsa graduate student at sdsu wants to measure the activity of a particular enzyme at ph 4.0. to buffer her reaction, she will use a buffer system based on one of the acids listed below, which acid is most appropriate for the experiment? of monosodium succinate (fw = 140 g/mol) and disodium succinate (fw = 162 g/mol) must be added to 1 l of water to produce a solution with a ph 5.28 and a total solute concentration of 100 mm? (assume the total volume remains 1 liter, answer in grams monosodium succinate, grams disodium succinate, respectively.) volumes of 0.05 m nah2po4 and 0.05 m na2hpo4 (pk's for phosphoric acid are 2.15, 6.82 and 12.38). which of the following best describes the resulting solution?
Answers: 2

Chemistry, 22.06.2019 14:50, chem1014
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2
Do you know the correct answer?
The phenolic indicator (in-oh)has approximately the same pka as a carboxylic acid. which h is the mo...
Questions in other subjects:



Mathematics, 23.09.2019 00:30




Social Studies, 23.09.2019 00:30


English, 23.09.2019 00:30

History, 23.09.2019 00:30