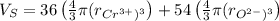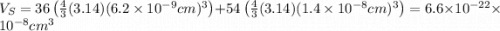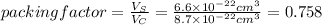Chemistry, 09.07.2019 22:00, nancye2008
The unit cell for cr2o3 has hexagonal symmetry with lattice parameters a = 0.4961 nm and c = 1.360 nm. if the density of this material is 5.22 g/cm3, calculate its atomic packing factor. the atomic weights of cr

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, brittanysanders
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 03:30, krharris
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 14:40, elawnnalewis4855
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2
Do you know the correct answer?
The unit cell for cr2o3 has hexagonal symmetry with lattice parameters a = 0.4961 nm and c = 1.360 n...
Questions in other subjects:

Geography, 02.06.2020 06:57

English, 02.06.2020 06:57


Mathematics, 02.06.2020 06:57



Mathematics, 02.06.2020 06:57

Mathematics, 02.06.2020 06:57

Mathematics, 02.06.2020 06:57

Mathematics, 02.06.2020 06:57


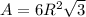
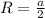
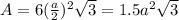
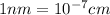
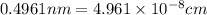
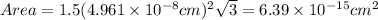
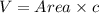
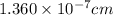
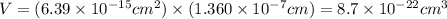
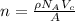
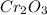 is 151.99 g/mol.
is 151.99 g/mol.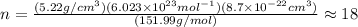
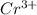 and
and 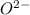 is 62 pm and 140 pm respectively.
is 62 pm and 140 pm respectively. 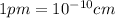
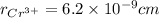
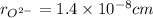
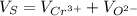
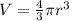 ,
,