

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:40, kellypechacekoyc1b3
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 04:30, akeemedwards12
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2

Chemistry, 22.06.2019 14:00, rosetoheart2
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2
Do you know the correct answer?
Calculate the mean free path of air molecules at 3.50 * 10-13 atm and 300 k. (this pressure is readi...
Questions in other subjects:


Mathematics, 20.10.2020 01:01

Mathematics, 20.10.2020 01:01




History, 20.10.2020 01:01


Mathematics, 20.10.2020 01:01

Mathematics, 20.10.2020 01:01


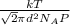 ---------(1)
---------(1)



