
Chemistry, 10.07.2019 01:30, KayleighMorganhopkin
At 503 k the equilibrium constant kc for the dissociation of n2o4 has the value of 40.0. calculate the fraction of n2o4 left undissociated when one mole of this gas is placed in a 1.0-l container at 503 k

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, Chente379
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2


Chemistry, 22.06.2019 19:00, ecolifesfsu1263
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1
Do you know the correct answer?
At 503 k the equilibrium constant kc for the dissociation of n2o4 has the value of 40.0. calculate t...
Questions in other subjects:

Social Studies, 09.10.2019 15:00

History, 09.10.2019 15:00

Mathematics, 09.10.2019 15:00




Mathematics, 09.10.2019 15:00

Mathematics, 09.10.2019 15:00

Mathematics, 09.10.2019 15:00

Mathematics, 09.10.2019 15:00


 is
is 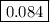 .
.
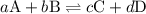
![{K_{\text{c}}} = \dfrac{{{{\left[ {\text{C}} \right]}^c}{{\left[ {\text{D}} \right]}^d}}}{{{{\left[ {\text{A}} \right]}^a}{{\left[ {\text{B}} \right]}^b}}}](/tpl/images/0071/4755/ca3d6.png)
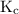 is the equilibrium constant.
is the equilibrium constant.
 is as follows:
is as follows:
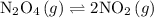
![\begin{aligned}\left[ {{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{4}}}} \right]&= \frac{{1{\text{ mol}}}}{{1{\text{ L}}}}\\&= 1{\text{ M}}\\\end{aligned}](/tpl/images/0071/4755/2a76f.png)
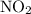 becomes 2x at equilibrium.
becomes 2x at equilibrium.
![{{\text{K}}_{\text{c}}}=\dfrac{{{{\left[ {{\text{N}}{{\text{O}}_2}} \right]}^2}}}{{\left[ {{{\text{N}}_2}{{\text{O}}_4}} \right]}}](/tpl/images/0071/4755/bd6a5.png) …… (1)
…… (1) ![\left[ {{\text{N}}{{\text{O}}_{\text{2}}}} \right]](/tpl/images/0071/4755/54e5f.png) , 1-x for
, 1-x for ![\left[ {{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{4}}}} \right]](/tpl/images/0071/4755/2dd51.png) and 40 for
and 40 for 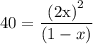 …… (2)
…… (2)

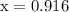
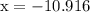
 …… (3)
…… (3) 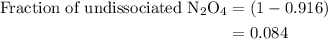
 (aq):
(aq):




