Chemistry, 10.07.2019 03:30, hallmansean04
For each of the acid–base reactions, calculate the mass (in grams) of each acid necessary to completely react with and neutralize 4.85 g of the base. a. hcl(aq) + naoh(aq) s h2o(l) + nacl(aq) b. 2 hno3(aq) + ca(oh)2(aq) s 2 h2o(l) + ca(no3)2(aq) c. h2so4(aq) + 2 koh(aq) s 2 h2o(l) + k2so4(aq)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, EinsteinBro
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 05:50, vanessa051266
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 22.06.2019 09:00, tashaunalewis4786
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3
Do you know the correct answer?
For each of the acid–base reactions, calculate the mass (in grams) of each acid necessary to complet...
Questions in other subjects:

Business, 11.07.2019 08:50

Advanced Placement (AP), 11.07.2019 09:00

Social Studies, 11.07.2019 09:00

Mathematics, 11.07.2019 09:00


Social Studies, 11.07.2019 09:00

Mathematics, 11.07.2019 09:00

Biology, 11.07.2019 09:00


Mathematics, 11.07.2019 09:00



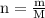
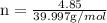
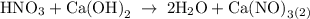
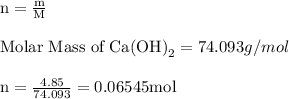



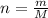
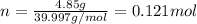
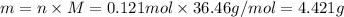
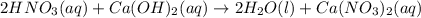
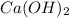 completely reacts with 2 mol of
completely reacts with 2 mol of 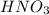 . The mass of
. The mass of 
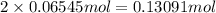 of
of 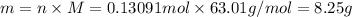
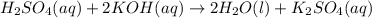
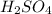 . The mass of KOH is given 4.85 g, convert this into number of moles as follows:
. The mass of KOH is given 4.85 g, convert this into number of moles as follows: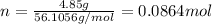
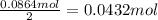 of
of