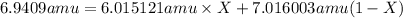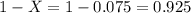Chemistry, 10.07.2019 03:30, Shihhschool20
Lithium forms compounds which are used in dry cells and storage batteries and in high-temperature lubricants. it has two naturally occurring isotopes, 6li (isotopic mass = 6.015121 amu) and 7li (isotopic mass = 7.016003 amu). lithium has an atomic mass of 6.9409 amu. what is the percent abundance of lithium-6?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, kaylaamberd
Write the complete balanced equation for the reaction between lead (iv) oxide (pbo2) and water (h2o).
Answers: 1

Chemistry, 22.06.2019 07:30, bbyniah123
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 07:50, mckinleesmomp6qj1e
Which of the following electromagnetic waves can create ions?
Answers: 2
Do you know the correct answer?
Lithium forms compounds which are used in dry cells and storage batteries and in high-temperature lu...
Questions in other subjects:

Health, 17.01.2022 18:40


Mathematics, 17.01.2022 18:40


Mathematics, 17.01.2022 18:40


Social Studies, 17.01.2022 18:40

Mathematics, 17.01.2022 18:40

Business, 17.01.2022 18:40