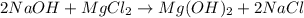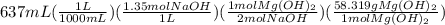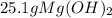Sodium hydroxide and magnesium chloride react as shown by this equation: 2naoh + mgcl2 → mg(oh)2 + 2nacl. suppose the reaction begins with 637 milliliters of 1.35 m sodium hydroxide solution and excess magnesium hydroxide. what is the theoretical yield of magnesium hydroxide if the resulting solution has a volume of 2.82 liters? use the periodic table and the polyatomic ion resource. the mass of magnesium hydroxide formed is grams

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 11:00, hannah5143
The human eye contains a molecule called 11-cis-retinal that changes shape when struck with light of sufficient energy. the change in shape triggers a series of events that results in an electrical signal being sent to the brain that results in vision. the minimum energy required to change the conformation of 11-cis-retinal within the eye is about 164 kj/mol.
Answers: 2

Chemistry, 22.06.2019 13:00, taylorpayne525p8qxky
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 18:00, AdoNice
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2
Do you know the correct answer?
Sodium hydroxide and magnesium chloride react as shown by this equation: 2naoh + mgcl2 → mg(oh)2 +...
Questions in other subjects:


Mathematics, 02.01.2022 04:00

Social Studies, 02.01.2022 04:10

History, 02.01.2022 04:10




Mathematics, 02.01.2022 04:10