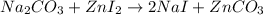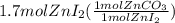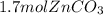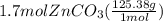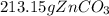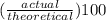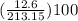Chemistry, 10.07.2019 07:30, takaralocklear
Areaction between 1.7 moles of zinc iodide and excess sodium carbonate yields 12.6 grams of zinc carbonate. this is the equation for the reaction: na2co3 + zni2 → 2nai + znco3. what is the percent yield of zinc carbonate? the percent yield of zinc carbonate is %.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:20, Naysa150724
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3


Chemistry, 22.06.2019 17:00, destinyycooper
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
Do you know the correct answer?
Areaction between 1.7 moles of zinc iodide and excess sodium carbonate yields 12.6 grams of zinc car...
Questions in other subjects:






Mathematics, 22.01.2021 18:30

Mathematics, 22.01.2021 18:30


History, 22.01.2021 18:30

History, 22.01.2021 18:30