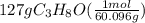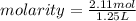Chemistry, 10.07.2019 07:30, sduhaime1974
Isopropanol (c3h8o) is a key ingredient in some hand sanitizers. suppose that 127 grams of isopropanol is dissolved in water. the volume of the solution is 1,250 milliliters. what is the molarity of the solution? refer to the periodic table to you answer. express your answer to three significant figures. the molarity of the solution is m.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:40, jude3412
In an effort to address concerns about global warming, a power plant in portland, oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 18:20, juansebas35
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Do you know the correct answer?
Isopropanol (c3h8o) is a key ingredient in some hand sanitizers. suppose that 127 grams of isopropan...
Questions in other subjects:

Physics, 27.10.2020 19:10


Computers and Technology, 27.10.2020 19:10



Geography, 27.10.2020 19:10

Arts, 27.10.2020 19:10

Mathematics, 27.10.2020 19:10

Geography, 27.10.2020 19:10

Geography, 27.10.2020 19:10


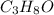 = 3(12.011) + 8(1.008) + 1(15.999) = 60.096 gram per mol
= 3(12.011) + 8(1.008) + 1(15.999) = 60.096 gram per mol