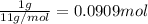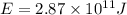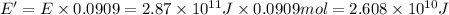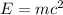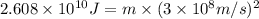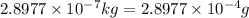Chemistry, 10.07.2019 09:00, ballin3294
Carbon-11 decays by positron emission: 116c → 115b + 01e the decay occurs with a release of 2.87 ⋅ 1011 j per mole of carbon-11. when 1.00 g of carbon-11 undergoes this radioactive decay, g of mass is converted to energy.

Answers: 1
Similar questions

Biology, 05.07.2019 04:30, Jaedenaleinson
Answers: 1


Biology, 15.10.2019 02:30, misswonderless
Answers: 2
Do you know the correct answer?
Carbon-11 decays by positron emission: 116c → 115b + 01e the decay occurs with a release of 2.87 ⋅...
Questions in other subjects:


English, 09.10.2019 15:50



Mathematics, 09.10.2019 15:50



Business, 09.10.2019 15:50

Mathematics, 09.10.2019 15:50


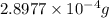 of mass is converted to energy.
of mass is converted to energy.