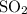Chemistry, 10.07.2019 17:00, kylieweeks052704
Part a choose the pair of substances that are most likely to form a homogeneous solution. choose the pair of substances that are most likely to form a homogeneous solution. co and c6h14 nh2ch3 and ch4 cbr4 and sf2 nf3 and so2 none of the pairs above will form a homogeneous solution.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:40, kellymcdow5135
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 10:00, alexabdercmur
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 10:00, melissa9882
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1
Do you know the correct answer?
Part a choose the pair of substances that are most likely to form a homogeneous solution. choose the...
Questions in other subjects:

Mathematics, 04.01.2020 01:31

Mathematics, 04.01.2020 01:31


Mathematics, 04.01.2020 01:31

Mathematics, 04.01.2020 01:31

Mathematics, 04.01.2020 01:31


Mathematics, 04.01.2020 01:31



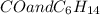 : CO is a polar gas and
: CO is a polar gas and 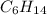 is a non polar liquid. Therefore, this solution will not be homogeneous.
is a non polar liquid. Therefore, this solution will not be homogeneous.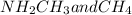 :
: 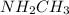 is a polar liquid while methane is a non polar gas. So, this solution will not be homogeneous.
is a polar liquid while methane is a non polar gas. So, this solution will not be homogeneous.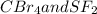 :
: 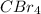 is a non polar solid while
is a non polar solid while 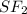 is a polar gas. So, they cannot form homogeneous solution.
is a polar gas. So, they cannot form homogeneous solution.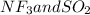 :
: 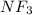 and
and 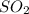 are both gases. So, they form a uniform homogeneous solution. Both can uniformly interact as they both are polar compounds.
are both gases. So, they form a uniform homogeneous solution. Both can uniformly interact as they both are polar compounds.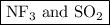 is the pair that is most likely to form a homogeneous solution.
is the pair that is most likely to form a homogeneous solution.
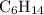
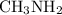 and
and 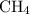
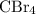 and
and 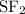
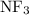 and
and