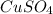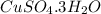Chemistry, 10.07.2019 21:00, saltedcaramel60
In another experiment, you have 4.5000 g of a copper(ii) sulfate hydrate with an unknown number of attached water molecules. after heating the hydrate, you have 3.3608 g of the anhydrous compound (copper(ii) sulfate with no waters) left. using these data, calculate the number of water molecules that is present in the formula of this hydrate (obviously before heating).

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, alevans7144
Why do sodium and neon have vastly different chemical and physical properties despite having similar atomic masses?
Answers: 2

Chemistry, 21.06.2019 21:00, alexmarche4675
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 21.06.2019 22:20, andybiersack154
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1

Chemistry, 22.06.2019 09:00, angelrenee2000
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2
Do you know the correct answer?
In another experiment, you have 4.5000 g of a copper(ii) sulfate hydrate with an unknown number of a...
Questions in other subjects:










History, 17.12.2019 01:31


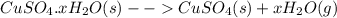
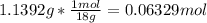
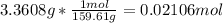
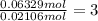
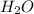 per one mol
per one mol