
The lab procedure involves several factors, listed below. some were variable and some were constant. label each factor below v for variable or c for constant. __ mass of the water in the calorimeter __ mass of the metal __change in temperature of the water __change in temperature of the metal __volume of water in calorimeter __calorimeter pressure __specific heat of water

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, jahmira96
Use this information to determine the number of calends electrons in the atoms. which of the following correctly compares the stability of the two atoms? a) both are unreactive b) both are highly reactive c) a is unreactive and d is reactive d) a is reactive and d is unreactive
Answers: 2

Chemistry, 22.06.2019 12:10, kaitlynbernatz2778
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 17:10, glitterpanda2468
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2
Do you know the correct answer?
The lab procedure involves several factors, listed below. some were variable and some were constant....
Questions in other subjects:


World Languages, 01.12.2020 23:10



Mathematics, 01.12.2020 23:10

Mathematics, 01.12.2020 23:10




Biology, 01.12.2020 23:10


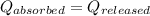
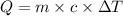
![m_1\times c_1\times \Delta T_1=-[m_2\times c_2\times \Delta T_2]](/tpl/images/0076/1361/825ce.png)
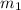 = mass of water in calorimeter
= mass of water in calorimeter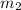 = mass of metal
= mass of metal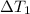 = change in temperature of the water
= change in temperature of the water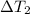 = change in temperature of the metal
= change in temperature of the metal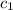 = specific heat of water
= specific heat of water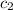 = specific heat of metal
= specific heat of metal



