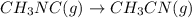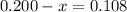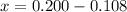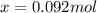Chemistry, 11.07.2019 07:00, aurelio1121
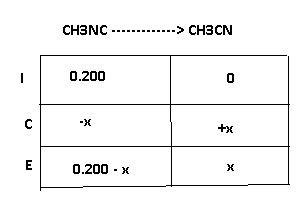
At elevated temperatures, methylisonitrile (ch3nc) isomerizes to acetonitrile (ch3cn): ch3nc (g) \rightarrow → ch3cn(g) at the start of an experiment, there are 0.200 mol of reactant and 0 mol of product in the reaction vessel. after 25 min, 0.108 mol of reactant (ch3nc) remain. there are mol of product (ch3cn) in the reaction vessel.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:00, rosie20052019
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1


Do you know the correct answer?
At elevated temperatures, methylisonitrile (ch3nc) isomerizes to acetonitrile (ch3cn): ch3nc (g) \r...
Questions in other subjects:

Spanish, 12.11.2020 01:30

Biology, 12.11.2020 01:30





Biology, 12.11.2020 01:30



Chemistry, 12.11.2020 01:30