
This reaction shows the complete combustion of octane, c8h18, a component of gasoline. 2 c8h18(l) + 25 o2(g) 16 co2(g) + 18 h2o(l) (a) how many moles of o2 are needed to burn 1.60 mol of c8h18? mol (b) how many moles of co2 are produced when 0.19 mol of c8h18 are burned? mol (c) how many grams of o2 are needed to burn 1.15 g of c8h18? g

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:40, wanderer3653
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3

Chemistry, 22.06.2019 18:30, kate3887
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1

Chemistry, 22.06.2019 21:20, jordan2875
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1
Do you know the correct answer?
This reaction shows the complete combustion of octane, c8h18, a component of gasoline. 2 c8h18(l) +...
Questions in other subjects:


Mathematics, 03.07.2019 07:00



Mathematics, 03.07.2019 07:00

Mathematics, 03.07.2019 07:00

Mathematics, 03.07.2019 07:00


Mathematics, 03.07.2019 07:00


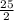 O₂→ 8 CO₂ + 9 H₂O.
O₂→ 8 CO₂ + 9 H₂O. 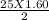 mole= 20 moles.
mole= 20 moles.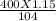 g= 4.42 g.
g= 4.42 g.



