
Chemistry, 11.07.2019 07:30, emilybutler
Sulfur and oxygen form both sulfur dioxide and sulfur trioxide. when samples of these were decomposed the sulfur dioxide produced 3.49 g oxygen and 3.50 g sulfur, while the sulfur trioxide produced 6.75 g oxygen and 4.50 g sulfur. calculate the mass of oxygen per gram of sulfur for each sample and show that these results are consistent with the law of multiple proportions.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:40, keiracoles
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1

Chemistry, 22.06.2019 17:30, katherineweightman
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1

Chemistry, 22.06.2019 21:00, rhondafits9000
Which property of water causes water drops to bead on a freshly waxed car?
Answers: 2

Chemistry, 23.06.2019 06:30, Liapis
An engineer decides to use a slightly weaker material rather than a stronger material, since she knows that the stronger material can break suddenly. this is an example of what? a choosing a material that will show warning before it fails b using composite materials that combine strength c using a material for multiple applications d using design techniques that increase efficiency and reduce cost
Answers: 3
Do you know the correct answer?
Sulfur and oxygen form both sulfur dioxide and sulfur trioxide. when samples of these were decompose...
Questions in other subjects:

Mathematics, 04.10.2021 05:40

English, 04.10.2021 05:40




Mathematics, 04.10.2021 05:40


Business, 04.10.2021 05:50

Mathematics, 04.10.2021 05:50


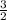 O₂
O₂



