
Chemistry, 11.07.2019 07:30, diamond8189
When 18.02 g of water is decomposed by electrolysis, 16.00 g of oxygen and 2.02 g of hydrogen are formed. according to the law of definite proportions. what mass, in grams, of hydrogen will be formed by the electrolysis of 795 g of water?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:40, markipler01
What is the study of how matter and energy interact? a. biology b. physics c. planetary science d. chemistry
Answers: 1

Chemistry, 22.06.2019 00:00, nyasiasaunders1234
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1


Chemistry, 22.06.2019 09:30, jewelz5887
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1
Do you know the correct answer?
When 18.02 g of water is decomposed by electrolysis, 16.00 g of oxygen and 2.02 g of hydrogen are fo...
Questions in other subjects:



Business, 09.11.2020 20:30

Mathematics, 09.11.2020 20:30


Chemistry, 09.11.2020 20:30


Physics, 09.11.2020 20:30



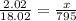
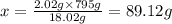
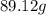 of hydrogen will be formed by the electrolysis of 795 g of water.
of hydrogen will be formed by the electrolysis of 795 g of water.



