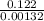Chemistry, 11.07.2019 11:30, taniyahbenyamin2
The decomposition reaction of a to b has a rate constant of 0.00132 s-1: a → 2b if the initial concentration of a is 0.156 m, how long will it take for the concentration of a to decrease by 78.1%

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, annsmith66
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1



Chemistry, 22.06.2019 21:30, emmalucilleblaha1995
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
Do you know the correct answer?
The decomposition reaction of a to b has a rate constant of 0.00132 s-1: a → 2b if the initial conc...
Questions in other subjects:


Mathematics, 13.06.2020 03:57


Physics, 13.06.2020 03:57


Mathematics, 13.06.2020 03:57

Biology, 13.06.2020 03:57

History, 13.06.2020 03:57

English, 13.06.2020 03:57

Mathematics, 13.06.2020 03:57


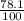 x 0.156
x 0.156