
Chemistry, 11.07.2019 15:00, wittlemarie
At a certain temperature, the solubility of n2 gas in water at 3.08 atm is 72.5 mg of n2 gas/100 g water . calculate the solubility of n2 gas in water, at the same temperature, if the partial pressure of n2 gas over the solution is increased from 3.08 atm to 8.00 atm . express your answer numerically to three significant figures.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, AIhunter2884
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 08:30, dyanaycooper13
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1


Chemistry, 22.06.2019 15:20, merrickrittany
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1
Do you know the correct answer?
At a certain temperature, the solubility of n2 gas in water at 3.08 atm is 72.5 mg of n2 gas/100 g w...
Questions in other subjects:



Mathematics, 19.01.2021 19:50

Mathematics, 19.01.2021 19:50

Mathematics, 19.01.2021 19:50

Engineering, 19.01.2021 19:50

Mathematics, 19.01.2021 19:50

SAT, 19.01.2021 19:50

Mathematics, 19.01.2021 19:50



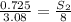
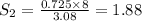
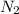 gas in 1 g of water or, 188 mg of tex]N_{2}[/tex] gas in 100 g of water.
gas in 1 g of water or, 188 mg of tex]N_{2}[/tex] gas in 100 g of water.



