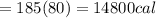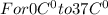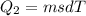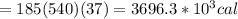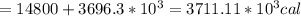Asports trainer applies an ice bag to the back of an injured athlete. calculate the heat in kcal that is absorbed if 185 g of ice at 0.0 ∘c is placed in an ice bag, melts, and rises to body temperature of 37.0 ∘ c. (for water, 80. cal (334 j) is needed to melt 1 g of ice or must be removed to freeze 1 g of water.)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, toledanomariap43bxm
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3


Chemistry, 22.06.2019 05:00, smartboy2296
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3
Do you know the correct answer?
Asports trainer applies an ice bag to the back of an injured athlete. calculate the heat in kcal tha...
Questions in other subjects:

Mathematics, 17.12.2020 20:30

English, 17.12.2020 20:30


Mathematics, 17.12.2020 20:30

Mathematics, 17.12.2020 20:30





Mathematics, 17.12.2020 20:30