
Chemistry, 11.07.2019 19:00, rainingsouls
You are presented with a white solid and told that due to careless labeling it is not clear if the substance is barium chloride, lead chloride, or zinc chloride. when you transfer the solid to a beaker and add water, the solid dissolves to give a clear solution. next a na2so4(aq) solution is added and a white precipitate forms. what is the identity of the unknown white solid?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, misspicafunpoke
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 22:00, robert7248
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 22.06.2019 22:30, wpatskiteh7203
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1
Do you know the correct answer?
You are presented with a white solid and told that due to careless labeling it is not clear if the s...
Questions in other subjects:

English, 23.12.2019 22:31


Mathematics, 23.12.2019 22:31


History, 23.12.2019 22:31

Social Studies, 23.12.2019 22:31




History, 23.12.2019 22:31


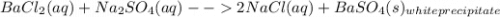

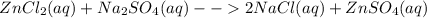
 + Na
+ Na
 2 NaCl + BaSO₄
2 NaCl + BaSO₄ 



