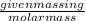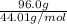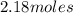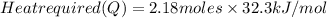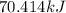Chemistry, 11.07.2019 19:00, kyrabrown33
Calculate the amount of heat required to completely sublime 96.0 g of solid dry ice (co2) at its sublimation temperature. the heat of sublimation for carbon dioxide is 32.3 kj/mol. calculate the amount of heat required to completely sublime 96.0 g of solid dry ice (co2) at its sublimation temperature. the heat of sublimation for carbon dioxide is 32.3 kj/mol.

Answers: 1
Similar questions


Chemistry, 17.09.2019 20:30, Orlandon220
Answers: 3


Chemistry, 16.10.2019 17:30, xlxJazmin
Answers: 1
Do you know the correct answer?
Calculate the amount of heat required to completely sublime 96.0 g of solid dry ice (co2) at its sub...
Questions in other subjects:

Mathematics, 29.10.2020 15:50

Social Studies, 29.10.2020 15:50

Mathematics, 29.10.2020 15:50

Mathematics, 29.10.2020 15:50


Mathematics, 29.10.2020 15:50



Mathematics, 29.10.2020 15:50

Biology, 29.10.2020 15:50


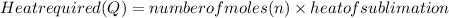 (1)
(1)