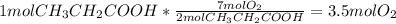Chemistry, 11.07.2019 19:00, nancylagunas805
__ ch3ch2cooh(l) + __ o2(g) __ co2(g) + __ h2o(l) how many moles of o2 are required to oxidize 1 mole of ch3ch2cooh according to the reaction represented above?

Answers: 1
Similar questions

Chemistry, 07.07.2019 21:40, TimothyYash6684
Answers: 2


Chemistry, 28.07.2019 22:00, ewalchloe5067920
Answers: 1
Do you know the correct answer?
__ ch3ch2cooh(l) + __ o2(g) __ co2(g) + __ h2o(l) how many moles of o2 are required to oxidize 1 m...
Questions in other subjects:

Social Studies, 08.01.2020 12:31

English, 08.01.2020 12:31

Mathematics, 08.01.2020 12:31



Mathematics, 08.01.2020 12:31




Mathematics, 08.01.2020 13:31


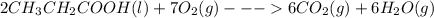
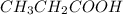 require 7 mol
require 7 mol 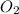 for complete combustion to produce
for complete combustion to produce 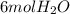 and
and