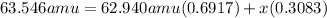Chemistry, 11.07.2019 19:30, oranjejuice
Copper has two naturally occurring isotopes and an atomic mass of 63.546 amu. cu-63 has a mass of 62.940 amu and an abundance of 69.17%. what is the identity and percent abundance of copper's other isotope? (type your answer for identity using the format cl-35 for chlorine-35.)

Answers: 1
Similar questions


Chemistry, 19.09.2019 16:30, xxaurorabluexx
Answers: 1


Chemistry, 24.10.2019 17:43, freeman36
Answers: 3
Do you know the correct answer?
Copper has two naturally occurring isotopes and an atomic mass of 63.546 amu. cu-63 has a mass of 62...
Questions in other subjects:


Mathematics, 20.04.2021 05:40

History, 20.04.2021 05:40



Mathematics, 20.04.2021 05:40


Chemistry, 20.04.2021 05:40

Law, 20.04.2021 05:40