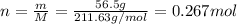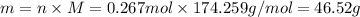Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:50, kaylamount
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 23.06.2019 03:00, makayyafreeman
Select the correct answer. wax is a nonpolar substance. in which type of substance is it the most soluble?
Answers: 1

Chemistry, 23.06.2019 03:10, 3jazybraxy
Which is true according to the law of conservation of energy
Answers: 1
Do you know the correct answer?
For each precipitation reaction, calculate how many grams of the first reactant are necessary to com...
Questions in other subjects:




Mathematics, 30.05.2020 01:57


Mathematics, 30.05.2020 01:57

Spanish, 30.05.2020 01:57

History, 30.05.2020 01:57

Mathematics, 30.05.2020 01:57

Mathematics, 30.05.2020 01:57


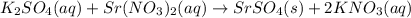
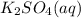 reacts with 1 mole of reactant 2
reacts with 1 mole of reactant 2 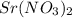 .
.