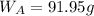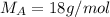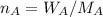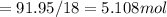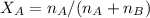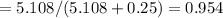A8.05 % ch3oh(aq) has a density of 0.976 g/ml at 18°c what is the mole fraction of the solvent in the solution? 0.953 8.05 91.95 0.0469
2) an aqueous solution of cesium chloride is prepared by dissolving 52.3 g cesium chloride in 60.0g of water at 25°c. the volume of this solution is 63.3 ml . what is the molality of the solution?
0.311 m
4.91 m
2.77 m
5.18 m
3) an aqueous solution of cesium chloride is prepared by dissolving 52.3 g cesium chloride in 60.0g of water at 25°c. the volume of this solution is 63.3 ml . what is the molarity of the solution?
4.91 m
2.69 m
5.18 m
2.77 m
4) the vapor pressure of ethanol, c2h5oh is 100.0 torr at 35 °c. calculate the vapor pressure of the solution formed by dissolving 28.8 g of alpha naphthol, c10h8o, in
36.8 g of c2h5oh. assume alpha naphthol to be nonvolatile at this temperature.
20.0 torr
43.9 torr
80.0 torr
56.1 torr
5) both ethanol, c2h5oh and propanol, c3h7oh, are volatile. at 35 °c, the vapor pressure of pure ethanol is 100 torr and that of propanol is 37.6 torr. what is the vapor pressure at this temperature of a solution is formed by mixing 36.9 g of ethanol and 12.0 g propanol.
15.3 torr
50.1 torr
84.7 torr
87.5 torr
the boiling point of pure ethanol, c2h5oh, is 78.4 latex: ^\circ ∘ c. its boiling point elevation constant is 1.22 °c/m. what is the boiling point of a solution formed by dissolving 8.00 g of alpha-naphthol (c10h7oh) in 100.0 g ethanol.
91.3 degrees centigrade
79.1 degrees centigrade
97.6 degrees centigrade
78.5 degrees centigrade
the freezing point of ccl4 is -22.92°c. calculate the freezing point of the solution prepared by dissolving 17.5g of pyrazine (c4h4n2) in 1250g of ccl4. the freezing point depression constant for ccl4 is 29.8 °c/m.
-22.50
-23.34
-17.71
-28.13
consider the following aqueous solutions:
a. 0.10 m nh4no3,
b. 0.10 m fe(no3)3
c. 0.10 m ba(no3)2
d. 0.10 m nh2conh2
arrange the following in increasing order (smallest to largest) order of osmotic pressure
c < b < a < d
a < d < c < b
d < a < c < b
a < c < b < d
consider the following aqueous solutions:
a. 0.10 m nh4no3,
b. 0.10 m fe(no3)3
c. 0.10 m ba(no3)2
d. 0.10 m nh2conh2
arrange the following in increasing order (smallest to largest) order of freezing point. the freezing point of pure water is 0.00 ∘ c and its freezing point depression constant is 1.86 ∘ c/m
d < a < c < b
b < c < a < d
c < b < a < d
a < c < b < d
consider the following aqueous solutions:
a. 0.10 m nh4no3,
b. 0.10 m fe(no3)3
c. 0.10 m ba(no3)2
d. 0.10 m nh2conh2
arrange the following in increasing order (smallest to largest) order of normal boiling point. the normal boiling point of pure water is 100.00 ∘ c and its boiling point elevation constant is 0.512 ∘ c/m
c < b < a < d
d < a < c < b
a < c < b < d
b < c < a < d
a solution is prepared by dissolving 1.22 g of compound in enough water to make up 262 ml in volume. the osmotic pressure of the solution is found to be 30.3 torr at
35.0 °c. calculate the molar mass of the compound.
257 g/mol
2950 g/mol
3.88 g/mol
44.7 g/mol
i tried to solve them all but i keep getting wrong answers can anyone

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:30, dorindaramirez0531
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1

Chemistry, 23.06.2019 00:00, maronetham6253
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2

Chemistry, 23.06.2019 04:20, milkshakegrande101
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1

Chemistry, 23.06.2019 12:30, floihqrrigweo
How do you interpret a chromatogram for what mixtures contain?
Answers: 3
Do you know the correct answer?
A8.05 % ch3oh(aq) has a density of 0.976 g/ml at 18°c what is the mole fraction of the solvent in th...
Questions in other subjects:

Social Studies, 18.05.2021 19:50

Spanish, 18.05.2021 19:50



Mathematics, 18.05.2021 19:50


Mathematics, 18.05.2021 19:50


Mathematics, 18.05.2021 19:50