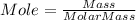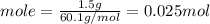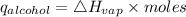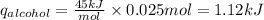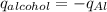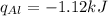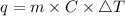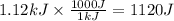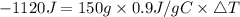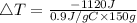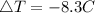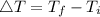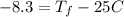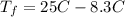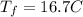Chemistry, 11.07.2019 22:30, reesewaggoner8
Consider 1.5g of rubbing alcohol (c3h8o) placed on a 150.g block of aluminum at 25 c. if all of the rubbing alcohol evaporates at 25 c, what is the final temperature of the aluminum?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:00, only1cache
Which is true concerning the products and reactants of photosynthesis and cellular respiration? a. the products of photosynthesis are sugars and the reactants of cellular respiration are starches. b. the products of photosynthesis are reactants in cellular respiration. c. oxygen is needed for photosynthesis and is given off in cellular respiration.
Answers: 2

Chemistry, 23.06.2019 03:00, amberskids2
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2
Do you know the correct answer?
Consider 1.5g of rubbing alcohol (c3h8o) placed on a 150.g block of aluminum at 25 c. if all of the...
Questions in other subjects: