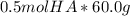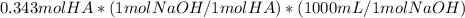Chemistry, 11.07.2019 22:30, lekaje2375
How would you prepare 2.00 l of a 0.25 m acetate buffer at ph= 4.50 from concentrated acetic acid (17.4 m) and 1.00 m naoh? the pka of acetic acid is 4.76?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, palcochran1313
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1


Chemistry, 22.06.2019 16:30, ccispoppin12
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 19:50, VoidedAngel
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3
Do you know the correct answer?
How would you prepare 2.00 l of a 0.25 m acetate buffer at ph= 4.50 from concentrated acetic acid (1...
Questions in other subjects:

Social Studies, 02.12.2020 17:20


Social Studies, 02.12.2020 17:20

Geography, 02.12.2020 17:20

Mathematics, 02.12.2020 17:20




Mathematics, 02.12.2020 17:20


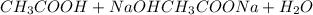
![[A-]/[HA] = 10^p^H^-^p^K^_a](/tpl/images/0078/7089/a3829.png)
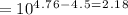
![[ A- ]+ [ HA ]= 0.5](/tpl/images/0078/7089/612df.png)
![[ A^- ] = 0.5 – [ HA]](/tpl/images/0078/7089/539a7.png)