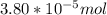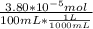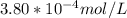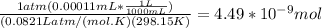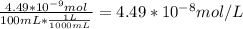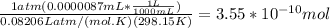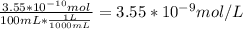A) a concentration of 5.24 ppm he means 5.24 μl of he per liter of air. using the ideal gas law in find how many moles of he are contained in 5.24 μl at 25.00°c (298.15 k) and 1.000 atm. this number is the molarity of he in the air. (2.11x10-7m) (b) find the molar concentrations of ar, kr, and xe in air at 25°c and 1 atm.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, monithebtslover01
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1

Chemistry, 23.06.2019 00:30, joshsmith2022
Five different substances are given to you to be dissolved in water. which substances are most likely to undergo dissolution in water? check all that apply. view available hint(s) check all that apply. sodium fluoride, naf octane, c8h18 propanol, ch3ch2ch2oh potassium iodide, ki benzene, c6h6
Answers: 1

Chemistry, 23.06.2019 01:30, kcarstensen59070
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1
Do you know the correct answer?
A) a concentration of 5.24 ppm he means 5.24 μl of he per liter of air. using the ideal gas law in f...
Questions in other subjects:




Mathematics, 04.02.2021 18:30

Arts, 04.02.2021 18:30



Mathematics, 04.02.2021 18:30

Spanish, 04.02.2021 18:30


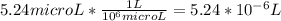
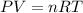
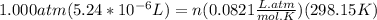
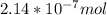
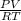
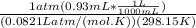 =
=