
(a) a 0.2 m potassium hydroxide solution is titrated with a 0.1 m nitric acid solution. (i) balanced equation: (ii)what would be observed if the solution was titrated well past the equivalence point using bromthymolblue as the indicator? (bromthymol blue is yellow in acidic solution and blue in basic solution.)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 23:30, emmalado45
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1

Chemistry, 23.06.2019 02:00, Robloxdemonduckyt
As light moves from one material into the next, which of the following affects how much the light waves will refract, or bend? angle at which the ray strikes the medium color of the material density of the material temperature of the light wave
Answers: 2


Chemistry, 23.06.2019 10:30, taniyahbenyamin2
What’s the physical properties in calcium chloride
Answers: 1
Do you know the correct answer?
(a) a 0.2 m potassium hydroxide solution is titrated with a 0.1 m nitric acid solution. (i) balanced...
Questions in other subjects:



History, 16.12.2021 04:40

Mathematics, 16.12.2021 04:40

Mathematics, 16.12.2021 04:40

History, 16.12.2021 04:40

History, 16.12.2021 04:40




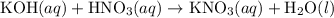
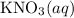 (and water) at the equivalence point. Both potassium hydroxide and nitric acid exist as strong electrolytes. As a result,
(and water) at the equivalence point. Both potassium hydroxide and nitric acid exist as strong electrolytes. As a result, 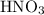 is added to the initially-basic solution. PH value of the solution would keep decreasing as the volume of the acid added increases. The final solution would be acidic as it contains not only water and
is added to the initially-basic solution. PH value of the solution would keep decreasing as the volume of the acid added increases. The final solution would be acidic as it contains not only water and 



