
Chemistry, 12.07.2019 01:30, nefertiri64
In a reaction where a + b ab, what does an equilibrium position of a= 14%, b = 14%, and ab= 72% indicate about the reaction? a. because a and b are equal, the reaction has reached equilibrium. b. there is more a and b than ab at equilibrium, and the reaction favors the reactants. c. there is more ab than a and b at equilibrium, and the reaction favors the products. d. there is more ab than a and b at equilibrium, and the reaction favors the reactants. e. the reaction is not at equilibrium.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:00, cnfndbxbfbdb2031
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 14:40, sugardime
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Do you know the correct answer?
In a reaction where a + b ab, what does an equilibrium position of a= 14%, b = 14%, and ab= 72% indi...
Questions in other subjects:



Mathematics, 14.01.2021 18:50



Mathematics, 14.01.2021 18:50

Mathematics, 14.01.2021 18:50


Arts, 14.01.2021 18:50

Arts, 14.01.2021 18:50


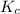 is defined as the equilibrium constant. It is defined as the ratio of concentration of products to the concentration of reactants.
is defined as the equilibrium constant. It is defined as the ratio of concentration of products to the concentration of reactants.
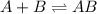
![K_c=\frac{[AB]}{[A][B]}](/tpl/images/0079/1606/5faa1.png)
![K_c=\frac{[0.72]}{[0.14][0.14]}=36.7](/tpl/images/0079/1606/2d664.png)
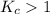 ; then the reaction is product favored.
; then the reaction is product favored.
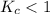 ; then the reaction is reactant favored.
; then the reaction is reactant favored.
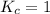 ; then the reaction is in equilibrium.
; then the reaction is in equilibrium.





