
Acompound contains only carbon, hydrogen, and oxygen. combustion of 9.612 mg of the compound yields 14.41 mg co2 and 3.93 mg h2o. the molar mass of the compound is 176.1 g/mol. what are the empirical and molecular formulas of the compound? (type your answer using the format co2 for co2.)

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:00, miguel454545
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 22:10, zwbaby3693
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 23.06.2019 06:00, asalimanoucha2v
•what conclusions can you make about the relationship between the volume of a gas and its temperature? • what conclusions can you make about the relationship between the volume of a gas and its pressure? • what possible variables have you not accounted for? as you did the procedures, is it possible that the atmospheric pressure may have changed? if it did change over the course of your experiment, then how would your results have been affected?
Answers: 3

Chemistry, 23.06.2019 08:30, alexiasommers41
Imagine you are a business executive who wants to pursue an environment policy for your company that limits pollution and uses fewer raw materials but would cost more what might be the discussion to your next broad meeting how would you make your case to your shareholders
Answers: 1
Do you know the correct answer?
Acompound contains only carbon, hydrogen, and oxygen. combustion of 9.612 mg of the compound yields...
Questions in other subjects:

Mathematics, 06.11.2020 02:50

Mathematics, 06.11.2020 02:50



Mathematics, 06.11.2020 02:50






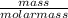 -(1)
-(1)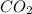 = 14.41 mg (given)
= 14.41 mg (given)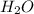 = 3.93 mg (given)
= 3.93 mg (given)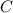 from
from 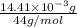
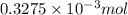
 from
from 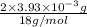
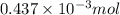
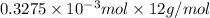
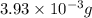
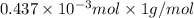
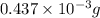
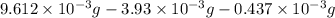
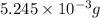
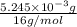
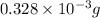
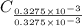
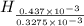
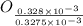
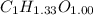
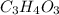
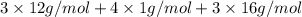
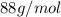
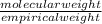 =
= 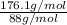
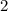
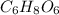 .
.




