
Chemistry, 12.07.2019 02:00, keviongardner
An aqueous solution that is 50.0 percent sulfuric acid (h2so4) by mass has a density of 1.143 g/ml. determine the molality of the solution.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, queenkimm26
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 06:30, dimondqueen511
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 09:00, stelllllllllllllllla
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1
Do you know the correct answer?
An aqueous solution that is 50.0 percent sulfuric acid (h2so4) by mass has a density of 1.143 g/ml....
Questions in other subjects:



Mathematics, 18.10.2020 07:01



Mathematics, 18.10.2020 07:01

Biology, 18.10.2020 07:01

Mathematics, 18.10.2020 07:01



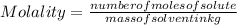 - (1)
- (1)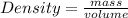 - (2)
- (2)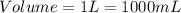
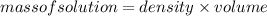
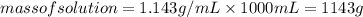

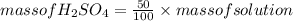
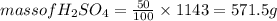
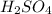 :
: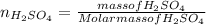
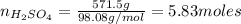
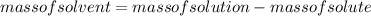
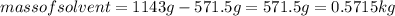
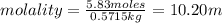
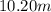 .
.




