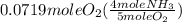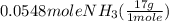Chemistry, 12.07.2019 02:30, Thejollyhellhound20
One of the steps in the commercial process for converting ammonia to nitric acid is the conversion of nh3 to no. 4 nh3(g) + 5 o2(g) → 4 no(g) + 6 h2o(g) in a certain experiment, 1.90 g of nh3 reacts with 2.30 g of o2. (a) which is the limiting reactant? o2 nh3 (b) how many grams of no and of h2o form? 1.73 g no 1.55 g h2o (c) how many grams of the excess reactant remain after the limiting reactant is completely consumed? g (d) show that your calculations in parts (b) and (c) are consistent with the law of conservation of mass. mass of products + excess reactant g total mass of reactants g

Answers: 1
Similar questions


Chemistry, 01.09.2019 09:30, yesseniaroman21
Answers: 1

Chemistry, 10.10.2019 18:50, gg808
Answers: 3

Chemistry, 26.10.2019 01:43, ethanyayger
Answers: 1
Do you know the correct answer?
One of the steps in the commercial process for converting ammonia to nitric acid is the conversion o...
Questions in other subjects:







Mathematics, 09.03.2020 08:45


Mathematics, 09.03.2020 08:46


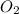 , (b) 1.73 g NO and 1.55 g
, (b) 1.73 g NO and 1.55 g 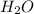 , (c) 0.932 g of
, (c) 0.932 g of 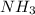 , (d) yes, the results are consistent with law of conservation of mass.
, (d) yes, the results are consistent with law of conservation of mass.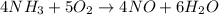
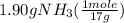 = 0.112 mole
= 0.112 mole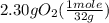 = 0.0719 mole
= 0.0719 mole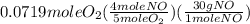 = 1.73 g of NO
= 1.73 g of NO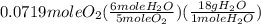 = 1.55 g of
= 1.55 g of