
Chemistry, 12.07.2019 02:30, jdkrisdaimcc11
Aluminum hydroxide reacts with sulfuric acid as follows: 2 al(oh)3(s) + 3 h2so4(aq) → al2(so4)3(aq) + 6 h2o(l). which reagent is the limiting reactant when 0.410 mol al(oh)3 and 0.410 mol h2so4 are allowed to react? h2so4 al2(so4)3 h2o al(oh)3 correct: your answer is correct. how many moles of al2(so4)3 can form under these conditions? 0.137 correct: your answer is correct. mol how many moles of the excess reactant remain after the completion of the reaction?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, soonerlady19
Which atom or ion is the largest? 0 a. 0 0 0 0 e. li
Answers: 2

Chemistry, 22.06.2019 10:10, andersonemma2222
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 12:40, carebear60
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1
Do you know the correct answer?
Aluminum hydroxide reacts with sulfuric acid as follows: 2 al(oh)3(s) + 3 h2so4(aq) → al2(so4)3(aq)...
Questions in other subjects:

English, 08.01.2020 09:31


Business, 08.01.2020 09:31



History, 08.01.2020 09:31

Biology, 08.01.2020 09:31


English, 08.01.2020 09:31


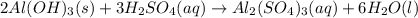
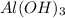 gives 1 mole of
gives 1 mole of 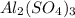 thus, 1 mole of
thus, 1 mole of 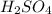 gives 1 mole of
gives 1 mole of 



