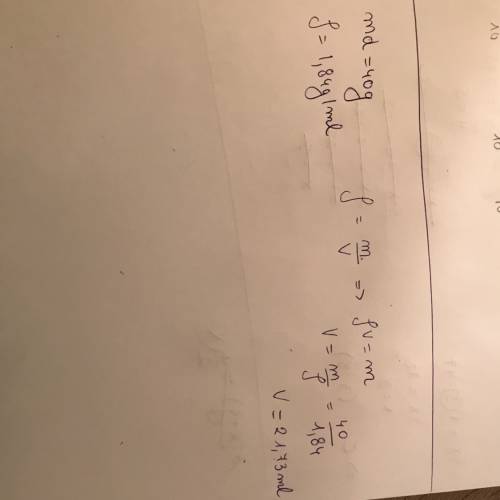Chemistry, 12.07.2019 05:30, KingJayDevil
Achemist needs 40.0 g of concentrated sulfuric acid for an experiment. the density of concentrated sulfuric acid at room temperature is 1.84 g/ml. what volume of the acid is required?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, vannitling12p4w44f
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1


Chemistry, 22.06.2019 14:30, Kiaraboyd9366
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1
Do you know the correct answer?
Achemist needs 40.0 g of concentrated sulfuric acid for an experiment. the density of concentrated s...
Questions in other subjects:





Computers and Technology, 11.11.2020 17:50