
Chemistry, 12.07.2019 05:30, christianconklin22
The gas no reacts with h2, forming n2 and h2o: $$2no(g)+2h2(g) 2h2o(g)+n2(g) if δ[no]/ δt = –24.0 m/s under a given set of conditions, what are the rates of change of [n2] and [h2o]?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, cicimarie2018
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 22.06.2019 00:20, TamB01
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 12:30, nekathadon
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1

Chemistry, 22.06.2019 18:30, rosie20052019
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1
Do you know the correct answer?
The gas no reacts with h2, forming n2 and h2o: $$2no(g)+2h2(g) 2h2o(g)+n2(g) if δ[no]/ δt = –24.0 m...
Questions in other subjects:







Social Studies, 02.08.2019 03:40




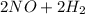 →
→ 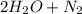 . For this reaction, rate of reaction is decrease in the concentration of NO with respect to time or decrease in the concentration of H₂ with respect to time or increase in the concentration of H₂O with respect to time or increase in the concentration of N₂ with respect to time.
. For this reaction, rate of reaction is decrease in the concentration of NO with respect to time or decrease in the concentration of H₂ with respect to time or increase in the concentration of H₂O with respect to time or increase in the concentration of N₂ with respect to time.![-\frac{1}{2} \frac{d[NO]}{dt}= -\frac{1}{2} \frac{d[H_{2} ]}{dt}=+\frac{1}{2} \frac{d[H_{2} O]}{dt}= +\frac{d[N_{2} ]}{dt}](/tpl/images/0079/8245/dff0b.png)
![\frac{d[NO]}{dt}=24.0 m/s](/tpl/images/0079/8245/5228b.png) . So, rates of change of [N₂]=
. So, rates of change of [N₂]= ![-\frac{1}{2} \frac{d[NO]}{dt}](/tpl/images/0079/8245/831c0.png) =
=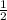 ×24.0 m/s=12 m/s. Rate of change of [H₂O] =
×24.0 m/s=12 m/s. Rate of change of [H₂O] =![+\frac{1}{2} \frac{d[H_{2} O]}{dt}=-\frac{1}{2} \frac{d[NO]}{dt}](/tpl/images/0079/8245/30264.png) = 24.0 m/s.
= 24.0 m/s.



