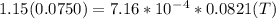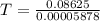Chemistry, 12.07.2019 11:30, ajayfurlow
A75.0-milliliter lightbulb is filled with neon. there are 7.16 × 10-4 moles of gas in it, and the absolute pressure is 116.8 kilopascals after the bulb has been on for an hour. how hot did the bulb get? the temperature of the lightbulb was k.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:10, cristinaledford3696
Imagine that you have produced several versions of lactase, each of which differs from normal lactase by a single amino acid. describe a test that could indirectly determine which of the versions significantly alters the three-dimensional shape of the lactase protein.
Answers: 2

Chemistry, 22.06.2019 11:50, bellojamilet410
What substance has a mass of 9.5g and volume of 2.1cm^3
Answers: 2

Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 16:30, Eddie997
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
Do you know the correct answer?
A75.0-milliliter lightbulb is filled with neon. there are 7.16 × 10-4 moles of gas in it, and the ab...
Questions in other subjects:

Mathematics, 13.08.2021 07:00

Mathematics, 13.08.2021 07:00



Mathematics, 13.08.2021 07:00

Mathematics, 13.08.2021 07:00


Mathematics, 13.08.2021 07:00

Mathematics, 13.08.2021 07:00


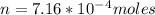
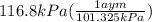
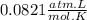 .
.