Chemistry, 12.07.2019 12:30, markmlg122
An unknown compound with a molar mass of 155.06 g/mol consists of 46.47% c, 7.80% h, and 45.72% cl. find the molecular formula for the compound. c6h12cl2 chcl c9h18cl3 c6h12cl

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, bobbycisar1205
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2


Chemistry, 22.06.2019 14:00, asanchez4292
What type of matter is made of only one kind of atom
Answers: 2

Chemistry, 22.06.2019 14:50, alexabbarker9781
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1
Do you know the correct answer?
An unknown compound with a molar mass of 155.06 g/mol consists of 46.47% c, 7.80% h, and 45.72% cl....
Questions in other subjects:



Social Studies, 30.05.2021 03:30



Mathematics, 30.05.2021 03:30





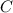 :
: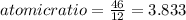
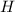 :
: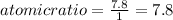
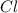 :
: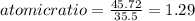
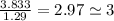
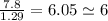
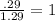
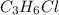 .
.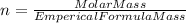
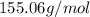

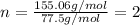
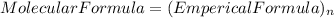 so, the molecular formula is:
so, the molecular formula is: