
Chemistry, 12.07.2019 14:30, pattydixon6
What is the empirical formula of an unknown compound that contains 60.3% of magnesium and 39.7% of oxygen?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:00, Angelanova69134
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 06:00, girly37
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 06:10, gabriellestaleyga16
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1
Do you know the correct answer?
What is the empirical formula of an unknown compound that contains 60.3% of magnesium and 39.7% of o...
Questions in other subjects:


English, 10.10.2019 15:50


Biology, 10.10.2019 15:50

History, 10.10.2019 15:50


Mathematics, 10.10.2019 15:50

Chemistry, 10.10.2019 15:50


History, 10.10.2019 15:50


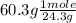 = 2.48 mole
= 2.48 mole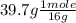 = 2.48 moles
= 2.48 moles



