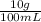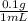Chemistry, 12.07.2019 15:00, hdwoody2002
The anesthetic procaine hydrochloride is often used to deaden pain during dental surgery. the compound is packaged as a 10.% solution (by mass; d = 1.0 g/ml) in water. if your dentist injects 0.30 ml of the solution, what mass of procaine hydrochloride (in milligrams) is injected?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, hannah2757
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 16:10, 00015746
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2


Chemistry, 23.06.2019 05:00, shealynh52
1. true or false: minerals are inorganic. true false 2. inorganic means that something has never been found alive 3. halite is another name for and is a mineral with a cubic crystal pattern. table salt rock salt
Answers: 2
Do you know the correct answer?
The anesthetic procaine hydrochloride is often used to deaden pain during dental surgery. the compou...
Questions in other subjects:



English, 13.05.2021 22:30

Health, 13.05.2021 22:30


English, 13.05.2021 22:30


Physics, 13.05.2021 22:30

Mathematics, 13.05.2021 22:30