
There are two binary compounds of mercury and oxygen. heating either of them results in the decomposition of the compound, with oxygen gas escaping into the atmosphere while leaving a residue of pure mercury. heating 0.6498 g of one of the compounds leaves a residue of 0.6018 g. heating 0.4172 g of the other compound results in a mass loss of 0.016 g. determine the empirical formula of each compound.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, luffybunny
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 16:30, montanolumpuy
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3


Chemistry, 22.06.2019 22:30, wpatskiteh7203
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1
Do you know the correct answer?
There are two binary compounds of mercury and oxygen. heating either of them results in the decompos...
Questions in other subjects:


Mathematics, 21.09.2019 21:30


Chemistry, 21.09.2019 21:30


Health, 21.09.2019 21:30

Social Studies, 21.09.2019 21:30




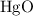 and
and 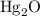 .
.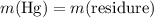
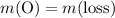
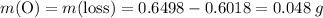
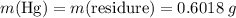
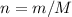 ;
; 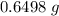 of the first compound would contain
of the first compound would contain 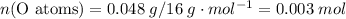
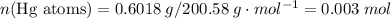
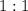 ratio; thus the empirical formula for this compound would be
ratio; thus the empirical formula for this compound would be 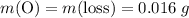
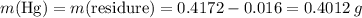
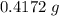 of the first compound would contain
of the first compound would contain 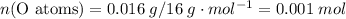
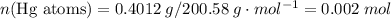
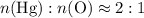 and therefore the empirical formula
and therefore the empirical formula



