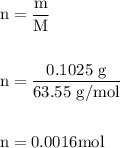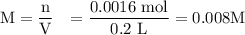Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, brittanygibson2812
Aballoon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Answers: 3

Chemistry, 21.06.2019 22:30, erinxmeow8
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons, neutrons, electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2


Do you know the correct answer?
A0.1025-g sample of copper metal is dissolved in 35 ml of concentrated hno3 to form cu2 ions and the...
Questions in other subjects:

History, 06.11.2020 02:10


History, 06.11.2020 02:10

Arts, 06.11.2020 02:10

Mathematics, 06.11.2020 02:10


Social Studies, 06.11.2020 02:10

Physics, 06.11.2020 02:10

Mathematics, 06.11.2020 02:10

Mathematics, 06.11.2020 02:10


 Cu(NO₃)₂ + NO₂ + H₂O
Cu(NO₃)₂ + NO₂ + H₂O