

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:20, mgavyn1052
Calculate the molarity of the solution. 6.02 x 1022 molecules of hci (molecular weight = 36.5 g/mole) in 2.0 liters of water m
Answers: 1


Chemistry, 22.06.2019 12:00, winterblanco
What is the lowest number energy level where a d sublevel is found
Answers: 1
Do you know the correct answer?
Titanium (ti) has an hcp crystal structure, a density of 4.51 g/cm3, and the atomic weight for ti, a...
Questions in other subjects:

History, 27.10.2021 22:20

Mathematics, 27.10.2021 22:20

Mathematics, 27.10.2021 22:20

Mathematics, 27.10.2021 22:20

English, 27.10.2021 22:20


Geography, 27.10.2021 22:20

Mathematics, 27.10.2021 22:20

Mathematics, 27.10.2021 22:20

Mathematics, 27.10.2021 22:20


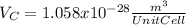
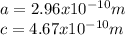
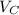 :
: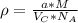
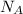 for the Avogadro number, thus:
for the Avogadro number, thus: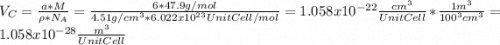
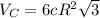
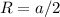 and
and 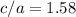 , thus:
, thus:![V_C=6c(a/2)^2\sqrt{3}\\V_C=6a*1.58(a/2)^2\sqrt{3}\\V_C=16.4a^3/4\\a=\sqrt[3]{\frac{4*1.058x10^{-28}\frac{m^3}{UnitCell}}{16.4} } \\a=2.96x10^{-10}m\\c=1.58a\\c=1.58*2.96x10^{-10}m\\c=4.67x10^{-10}m](/tpl/images/0081/7540/a224e.png)




