

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, itzyagirlshy
Determine the number o moles of ions/atoms/particle in the following: 2.50 miles of k2s (let me know how to do)
Answers: 1

Chemistry, 22.06.2019 02:50, JuniperGalaxy
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1

Chemistry, 22.06.2019 06:10, andybiersack154
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 15:00, raeprince9213
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1
Do you know the correct answer?
Cadmium metal reacts vigorously with yellow crystals of sulfur to produce a yellow powder of cadmium...
Questions in other subjects:







Social Studies, 14.12.2021 21:30




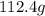 of cadmium with
of cadmium with 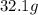 of sulfur =
of sulfur = 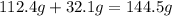
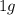 of cadmium forms
of cadmium forms 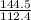 cadmium sulfide.
cadmium sulfide.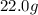 of cadmium will give
of cadmium will give 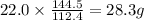 of cadmium sulfide.
of cadmium sulfide.




