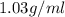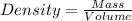Chemistry, 12.07.2019 22:00, jewelz5887
Magnesium (mg) is a valuable metal used in alloys, in batteries, and in the manufacture of chemicals. it is obtained mostly from seawater, which contains about 1.30 g of mg for every kilogram of seawater. calculate the volume of seawater (in liters) needed to extract 5.33 × 104 tons of mg. seawater has a density of 1.03 g/ml. (1 ton = 2000 lb; 1 lb = 453.6 g) enter your answer in scientific notation.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, BornAdopted21
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 13:50, amandamac7339
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Do you know the correct answer?
Magnesium (mg) is a valuable metal used in alloys, in batteries, and in the manufacture of chemicals...
Questions in other subjects:

Mathematics, 12.03.2021 18:30


Mathematics, 12.03.2021 18:30

English, 12.03.2021 18:30

Mathematics, 12.03.2021 18:30



Mathematics, 12.03.2021 18:30



 into gram.
into gram.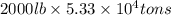
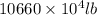 =
= 

 =
= 
 of seawater.
of seawater. =
= 
 of seawater.
of seawater.